Eliminates Contract Sterilization Facilities
Eliminates Contract Sterilization Facilities
- Shipping logistics associated with regional sterilization facilities
- Use of Tyvek
- 3rd party costs/fees

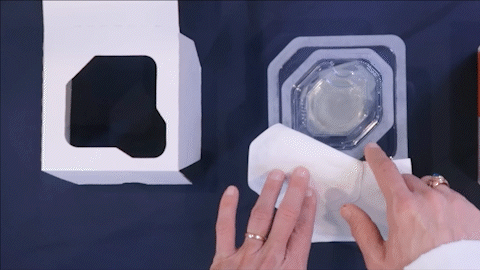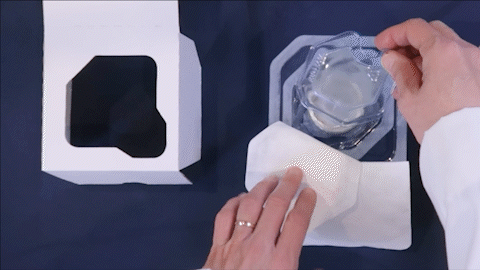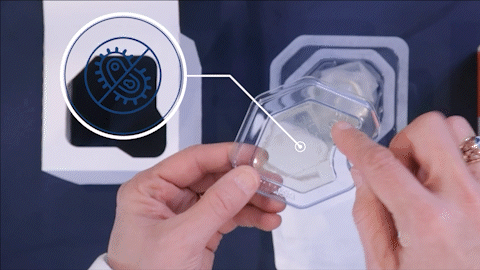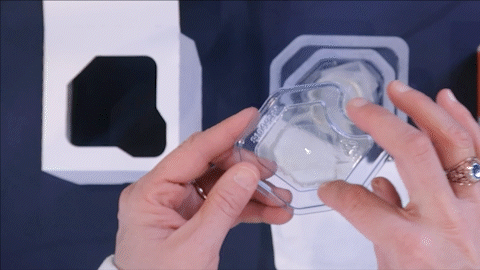
Eliminates EtO (Ethylene Oxide)
Eliminates EtO (Ethylene Oxide)
- EtO is a toxic chemical and a known carcinogen
- EPA has closed EtO sterilization facilities, will close more. FDA asked for alternatives to EtO.

Safer, Faster, Easier Sterilization
Safer, Faster, Easier Sterilization
- Safer for end-users and communities near EtO facilities
- Faster sterilization processing times
- Easier for manufacturers; no 3rd party sterilization logistics/costs